PureDEL technology
a revolutionary DEL platform
We have conceptualized, patented, and established a method for the production of
Self-Purified DNA-Encoded Libraries ("PureDEL"). [1]
Today, DNA-encoded chemical libraries (DEL) are generally synthesized in solution by split-and-pool methodology (Figure 1). DEL synthesis starts from a scaffold-DNA conjugate which is split in different wells for the attachment of the first set of chemical building blocks (BB) (e.g. 500 wells for 500 distinct BB). Following the chemical reactions, a distinct DNA fragment is ligated to each DNA-conjugate in order to encode each BB (i.e. 500 distinct codes for 500 BB). After this, step 1 of DEL synthesis is complete and all compounds are pooled together. A second round of splitting, attaching BB, encoding, and pooling can be performed, followed by further rounds. Generally, DELs are only synthesized with a maximum of 3–4 BB incorporation steps, as the overall yield decreases rapidly with an increasing number of consecutive reactions. Additionally, there is no quality control method to assess the purity of small molecules displayed in a DEL. While the DNA barcodes can be amplified by PCR and sequenced, the small molecules’ integrity cannot be assessed as they are present in very small quantities and in mixtures.
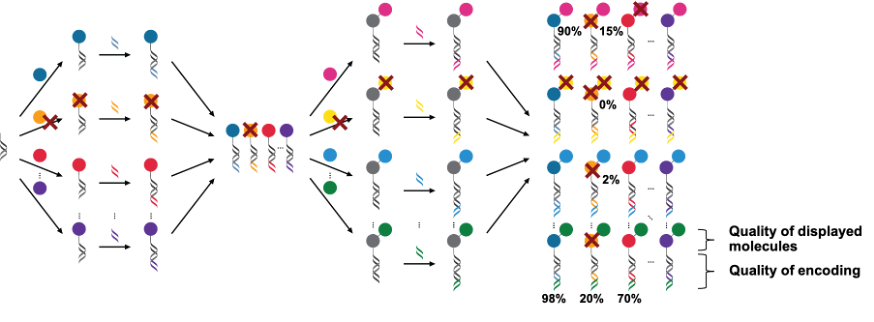
In Figure 1, as an example, the reactions to certain BB proceed with low yield, which results in a final library with a portion of the library members only displaying the desired molecule on a low percentage of the corresponding DNA barcodes.
In DEL selections, the quality of a DEL is absolutely key for performance. Binding ligands are identified by enrichment over the background in terms of sequencing counts. For instance, if a binding molecule is not synthesized readily such that only 2% of the DNA barcodes for this molecule actually present the correct molecule, in DEL selections, this molecule might be missed because the enrichment could still appear low. This limits DEL technology in the nature of BB which can be incorporated, in the number of consecutive reaction steps. Additionally, tedious HPLC purification of individual molecules after step 1 is usually performed to improve the quality of the DEL. At a later point in time, HPLC purification cannot be performed for individual molecules anymore, as the library members are present in mixtures.
Most importantly, these flaws ultimately limits maximal library size that can be successfully screened to typically 107-108.
To create a platform technology for challenging targets, and to overcome the above-mentioned limitations of DEL, we have established a method for DEL preparation based on solid phase synthesis (rather than the standard in solution) and developed a self-purification method allowing for inherent purification after the final synthesis step [WO2022084486], “PureDEL”. With self-purification, it is possible to include many BB positions while ensuring high library purity.
The PureDEL synthesis on optimized solid support enables the facile preparation of encoded molecules, as any excess reagents can simply be washed away. Additionally, reactions can be performed in neat organic solvent (rather than aqueous solution for conventional DEL) which allows for new DNA-compatible chemical reactions, without any DNA damage even in very harsh conditions (usually always some extent of DNA damage for conventional DEL), so new types of BB can be incorporated. The synthetic conversions observed are generally much better than for the established DEL synthesis. With our optimized reaction and ligation conditions, we can access a wide variety of chemical reaction types to make for the first time structurally diverse and drug-like encoded molecules from a chemical space ranging from small to intermediate-sized molecules.
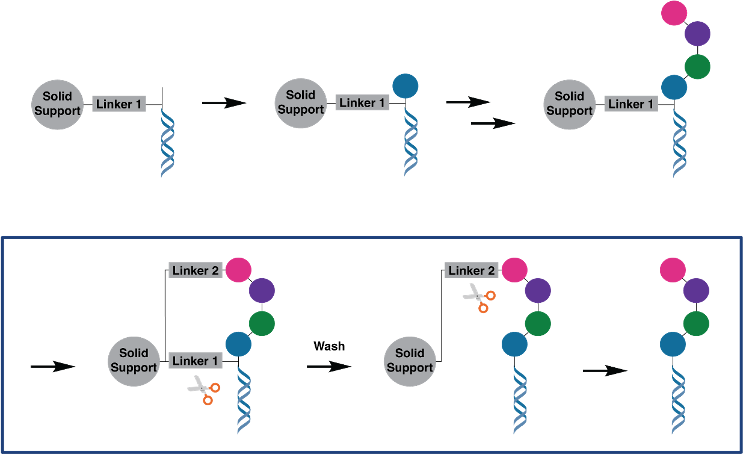
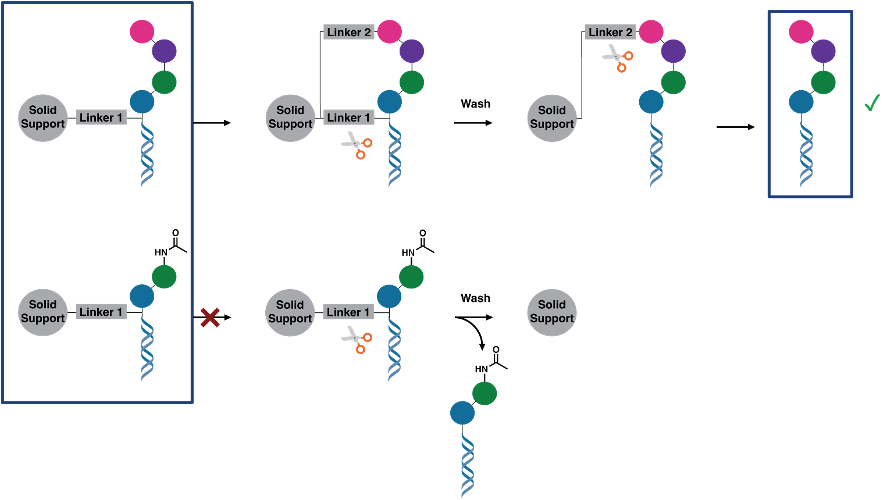
We have developed self-purification procedures to inherently purify the molecules after the final synthetic step. We have shown that impurities and incompletely synthesized intermediates can be reliably removed in this procedure. In the 2-linker self-purification approach, the DNA-encoded molecule is synthesized starting from a scaffold with a DNA tag attached to the solid support with a first linker (Figure 2). After split-and-pool synthesis to attach the BB and DNA barcodes (Figure 2, top row), a second linker (orthogonally cleavable) is installed between the final BB and the solid support. This second linker may only be installed if the molecule is completely synthesized, because after every BB incorporation step, a capping step is performed so that incomplete molecules cannot partake in further reactions. When the first linker is cleaved in the next step, all incompletely synthesized molecules are washed away, while the pure compounds are retained on the solid support through the second linker. In a final step, the pure DEL can be collected by cleavage of the second linker (self-purification shown in Figure 2, bottom row). Encoded compounds can for the first time include many BB positions while maintaining high quality to ensure performance in selections. Not only linear, but also macrocyclic, bicyclic or branched molecules can be prepared.
The purification of a mixture of completely and incompletely synthesized DNA-encoded compounds is shown in Figure 3.
While PureDEL technology, due to the solid-phase based synthesis can provide also purity-enhanced "standard" DELs composed of 2-4 sets of chemical building blocks, the unique selling position of PureDEL technology is that it is the first encoded display method for diverse intermediate-sized molecules, including macrocycles. PureDEL provides access to a new chemical space, with high-purity display. There is no restriction by the natural translation machinery as for phage or mRNA display, such that it is possible to perform reactions which yield molecules with drug-like properties focusing on cell permeability, proteolytic stability, oral availability and favorable biodistribution. PureDEL yields pure encoded molecules for reliable selection results.
[1] Keller, M.#, Petrov, D.#, Gloger, A., Dietschi, B., Jobin, K., Gradinger, T., Martinelli, A., Plais, L., Onda, Y., Neri, D., & Scheuermann, J.* (2024). "Highly pure DNA-encoded chemical libraries by dual-linker solid-phase synthesis" Science (New York, N.Y.), 384(6701), 1259–1265. external page https://doi.org/10.1126/science.adn3412